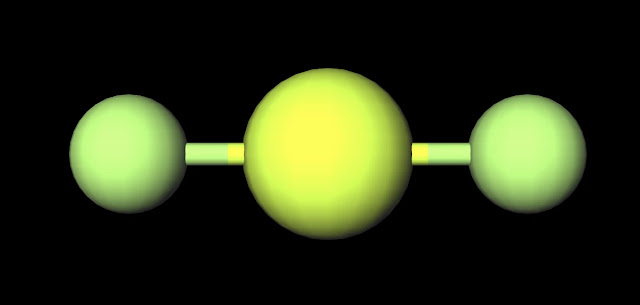
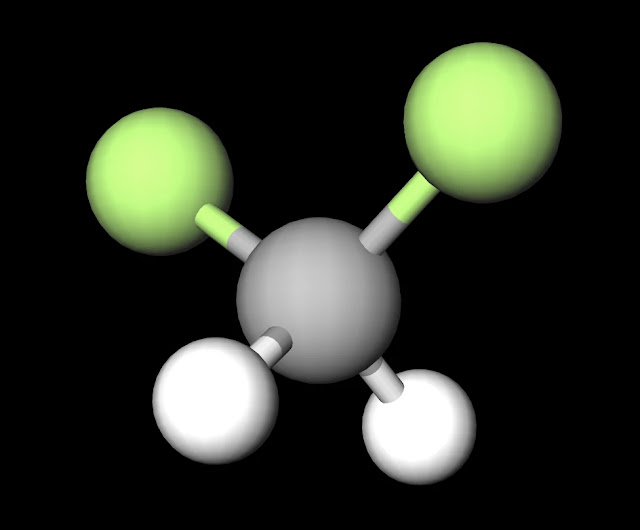
The dipole nature of this molecule is increased by the large electronegativity difference between oxygen (3.44) and hydrogen (2.20). This qualifies the bonds as polar covalent and creates partial charges as strong as those in water (H2O). Both oxygen molecules have two lone pairs which increases the amount of negative charge that appears around the center of this molecule. This is why H2O2 is liquid that has a slightly higher boiling point than water of 150˚C and is slightly more viscous (i.e. imagine that is is slightly harder to "swim" through this substance than water although you would never attempt to do this due to the reactive nature of H2O2). This property is also increased as a result of hydrogen peroxide's polar nature.
Another interesting fact relates to the spatial geometry of the molecule. While the oxygen's keep the hydrogens (since these are more positive and opposite partial charges attract) at a distance of approximately 100 pm, the two oxygens maintain of a distance of 150 pm, approx. 1.5 times that of the distance b/t oxygen and hydrogen. This distance again reinforces the true magnitude of negative charge within the center of this molecule.
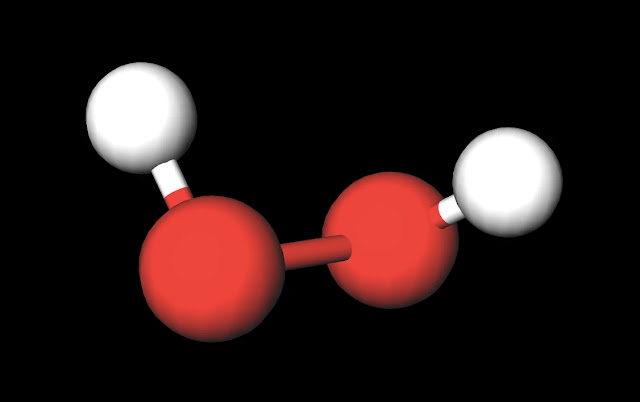 |
| H2O2 Ball and Stick Model. Created with MolView. |
H2O2 is commonly utilized for bleaching, disinfecting specific surfaces, and laundering. This is b/c H2O2 commonly reacts with and tags organic molecules, allowing them to be easily removed in wastewater treatment processes. This is also why it is very effective at destroying a wide range of pathogens including bacteria and viruses. However, approx. 60% of all hydrogen peroxide utilized goes to bleaching different items in industrial processes.