Since the difference between the electronegativity of sulfur (2.55) is quite small when compared to carbon (2.55), the bonds are nonpolar covalent leading to few charge differences within the actual molecule. Without a permanent pull of electrons one way or the other the molecule lacks a mechanism to create dipole-dipole interactions. Furthermore, the molecule has a linear structure which means that any possible small charges are also cancelled out across the molecule. The two sulfur atoms are symmetrical to each other, leading to them cancelling out any average charge and again removing the possibility of strong dipole-dipole interactions.
This is a similar mechanism to how CO2 is polar. Feel free to also check out our article about the Lewis Dot Structure for CO2 to learn more about CS2's more important counterpart. Oxygen and Sulfur have similar bonding patterns because they are both Chalcogens, having six valence electrons and being able to form two possible bonds (i.e. two single bonds or one double bond).
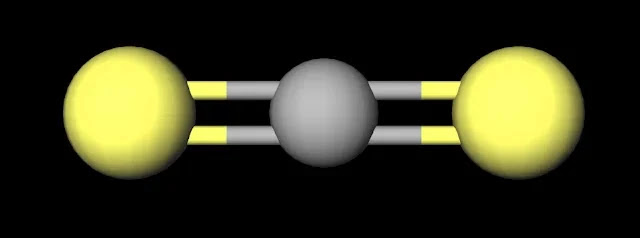 |
| CS2 Ball and Stick Model. Created with MolView. |
At standard temperature and pressure (STP) CO2 is a gas because of its nonpolar nature. CS2 on the other hand is a liquid at standard temperature and pressure although its melting point of 45˚C [1] isn't to much greater than the 25˚C standard at STP. CS2 is a liquid because of temporary charge interactions formed by London Dispersion Forces (i.e. the random movement of electrons). This is due to the larger number of electrons within CS2, specifically within the sulfur atoms when compared to the oxygen atoms within CO2. CS2 has a few niche uses within certain refining protocols as a solvent or intermediate molecule.
[1] https://pubchem.ncbi.nlm.nih.gov/compound/Carbon-disulfide
No comments:
Post a Comment