 |
| Created by MakeTheBrainHappy |
The lewis dot structure of CO2 gives it some unique properties. Since there are no lone pairs on the atom, it is a linear structure which makes the charges cancel it. Therefore it is nonpolar and relatively unreactive. These properties in addition to its small state makes it so that carbon dioxide has a low melting point and is mostly in the gaseous phase at STP (Standard Temperature and Pressure). Even though Carbon Dioxide is quite small, its density is still 1.67 times that of air.
 |
| A solid block of CO2. The IMF (intermolecular) forces holding this structure together are so weak that the cube is going directly from the solid to the gaseous phase. CO2 is solid at -78.5 ˚C. Source |
Yes, although these would not be the most common structures due to formal charges. You can learn more about what formal charge is in this article. An example of a possible resonance structure would be to make the double bonds in the lewis dot structure single bonds and then give the extra electrons to each of the oxygen atoms. This would give each oxygen atom a -1 formal charge and the carbon atom a +2 formal charge. While this would technically balance out, it would be unlikely due to the covalent nature of the bonds even though the bonds themselves are polar.
Furthermore, other lewis dot structures are highly unlikely due to the increased instability which results from the placement of formal charges. When something has charge it is more likely to interact with the environment in a way that will tend towards a more stable configuration. Consider this to be an extension of the first and second law of thermodynamics; namely, that molecules tend towards lower energy states. Adding charges means that you would need to input energy in order to maintain the charge difference; energy which simply isn't available.
Does carbon usually form bonds like this?
Yes, carbon combined with another element usually creates non polar structures because carbon can form multiple bonds and rarely has lone pair electrons. It can easily fill its octet requirement with almost any combination of elements and its electronegativity allows it bond with many different types of atoms. The notable exceptions are the compounds CN (cyanide) and CO (carbon monoxide), which the bond is polar due to the unequal sharing of electrons between the two atoms in the structure. The structures do not look like the lewis dot structure for CO2, where the carbon does not have any lone pair electrons on the carbon.
 |
| The Lewis Structure for Carbon Monoxide with lone pair electrons on the carbon. Source |
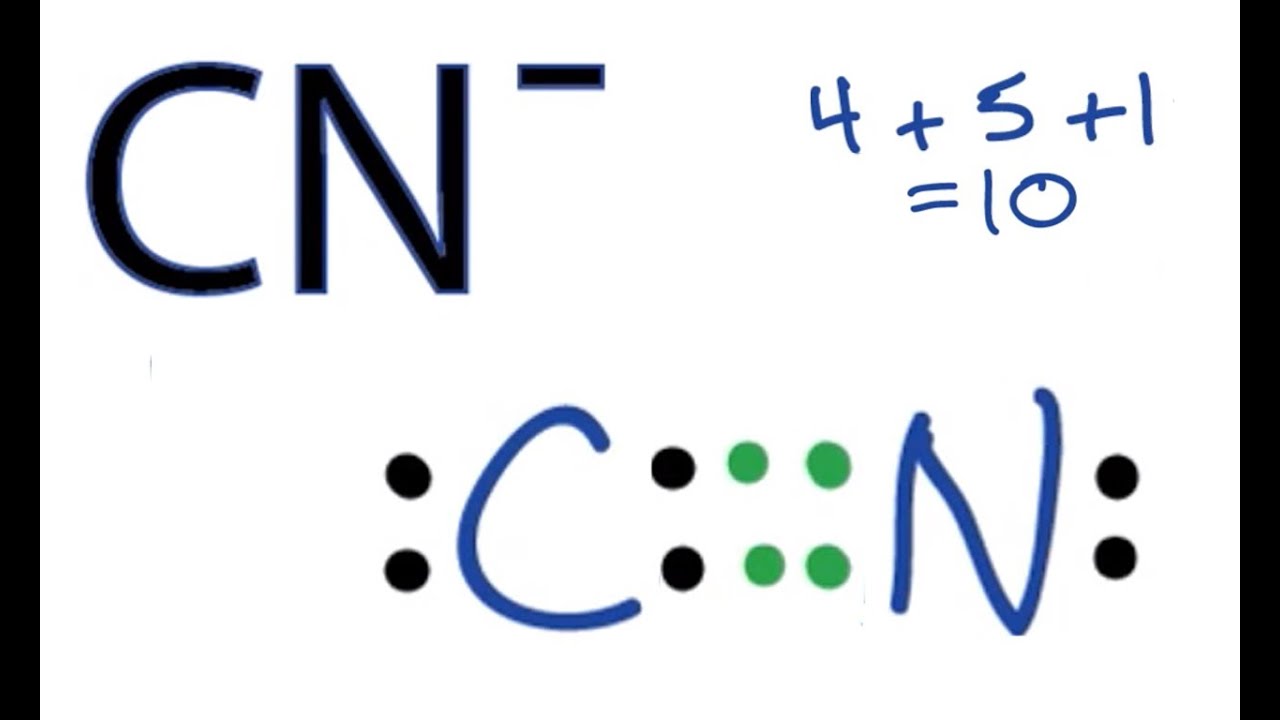 |
| The Lewis Structure for Cyanide with lone pair electrons on the carbon. Source |
If you are interested in learning more about the polarity of CO2 we highly recommend checking out our article regarding this subject. This will discuss trends which are already illuminated above in the lewis dot structure for CO2. Additionally there is a discussion of one of the most important applications of carbon on this planet (hint: the carbon cycle).
 |
| Solubility vs. increased pressure for carbon dioxide in pure water at 373.15K (source below) |
How should we interpret the graph?
 |
| How pressure impacts solubility for gaseous phase components. Source |
As the pressure increases more gaseous molecules are pressed against the surface of the liquid. This naturally means that more molecules will become dissolved liquid since dissolution rate is directly related to the amount of contact that the gas particles have with the water. In our case we can see that the solubility of CO2 increases for the same reason as pressure increases.
 |
| Table of Mole Fractions for the dissolution of Carbon Dioxide in Acetone. (Source below) |
Above you can see a table of mole fractions and they are far greater than the roughly 1% we saw with CO2 into water. At comparable pressures approx. 50-67% of the CO2 in dissolved in acetone. However the temperatures also have an impact on the solubility as he can be seen in thee table above (generally the values are lower at 333.15K as opposed to 313.15K).
 |
| How different solutions are impacted by temperature increases. Source |
 |
| Molecular Orbital Diagram for CO2. Source |
This model tries to incorporate many more observations from the lab which are difficult to explain/glossed over within the simpler models such as the Lewis Dot structure. While we do not need to get into the intricacies, the evolution of our models represents an increased understanding of how these chemical systems function. Nevertheless, we can get incredibly far as we have seen simply by utilizing observations based on the lewis dot structure for CO2.
In simple terms, the molecular orbital model for carbon dioxide (CO₂) helps us understand how the atoms in CO₂ interact with each other to form a molecule.
Carbon dioxide is composed of one carbon atom (C) and two oxygen atoms (O). The molecular orbital model describes how the electrons from these atoms arrange themselves to create the CO₂ molecule.
In this model, we imagine that the atomic orbitals of the carbon and oxygen atoms combine to form new molecular orbitals that spread out across the entire molecule. These molecular orbitals can hold the electrons that belong to the atoms.
In CO₂, the carbon atom's two outermost electrons (valence electrons) occupy its 2s and 2p atomic orbitals. The two oxygen atoms each have six valence electrons, which occupy their 2s and 2p atomic orbitals.
When the carbon and oxygen atoms come together to form CO₂, their atomic orbitals overlap and combine to create new molecular orbitals. Two molecular orbitals of particular importance are the sigma (σ) and pi (π) bonds.
Sigma (σ) Bond: The first molecular orbital formed is the sigma bond (σ bond). It results from the direct overlap of the 2s orbital of carbon with the 2p orbitals of the two oxygen atoms. This sigma bond holds the carbon and oxygen atoms together along the axis between them.
(C) (O) (O) | | | sigma sigma sigma bond bond bond
Pi (π) Bond: The second molecular orbital is the pi bond (π bond). It arises from the sideways overlap of the p orbitals of carbon and oxygen atoms that are perpendicular to the sigma bond.
(C) (O) (O) | | | pi pi pi bond bond bond
In carbon dioxide, the sigma bond and two pi bonds together hold the carbon and oxygen atoms together as a stable molecule. The double bonds (σ + π) between carbon and oxygen make CO₂ a linear molecule.
What are the applications of CO2 in a chemical context?
CO₂ is a versatile compound with various uses in chemistry, and here are some important applications:
Green Chemistry: CO₂ is a significant greenhouse gas, and there is a growing emphasis on using it as a sustainable feedstock in chemical reactions. As a chemistry PhD student, you may study and develop innovative catalytic processes to convert CO₂ into valuable chemicals, such as methanol or formic acid, through reduction reactions.
Supercritical CO₂: CO₂ under supercritical conditions (high temperature and pressure) exhibits unique properties, acting as a solvent with gas-like diffusivity and liquid-like density. This characteristic makes supercritical CO₂ an attractive medium for extraction processes, such as extracting natural products or separating organic compounds.
Carbon Capture and Storage (CCS): CCS technologies aim to capture CO₂ emissions from industrial processes or power plants, compress it, and store it underground to mitigate climate change. As a chemistry PhD student, you may research new materials for capturing CO₂ and investigate safe storage options.
CO₂ in Chemical Synthesis: CO₂ can be incorporated into chemical reactions, leading to the synthesis of various products. For instance, CO₂ is used in the synthesis of urea, salicylic acid, and other organic compounds.
Carbonation Reactions: CO₂ plays a crucial role in carbonation reactions, such as the production of carbonated beverages and the manufacturing of carbonate minerals and materials.
CO₂ as a Solvent: CO₂ can act as a green solvent for certain reactions and is used in supercritical fluid extraction to replace harmful organic solvents.
Polymer Synthesis: CO₂ can be utilized in polymerization reactions to produce polycarbonates and other polymers with unique properties.
CO₂ Sensors: Researchers may work on developing CO₂ sensors based on various principles, such as fluorescence, conductivity, or absorption, for environmental monitoring or medical applications.
CO₂ in Analytical Chemistry: CO₂ is used in gas chromatography as a mobile phase, and it is employed in infrared spectroscopy to detect CO₂ emissions and quantify its concentration in various samples.
CO₂ as a Reaction Promoter: CO₂ can act as a reactant or a reaction promoter in some organic reactions, facilitating the synthesis of specific compounds.
These applications demonstrate that CO₂ is not only a greenhouse gas with environmental implications but also a valuable resource with significant potential for sustainable chemistry and various industrial processes.
What are the applications of CO2 in a biological context?
Here are some key applications of CO₂ in biology:
Cell Culture: In cell culture laboratories, carbon dioxide is used in CO₂ incubators to maintain the optimal pH of cell culture media. The CO₂ gas dissolves in the media, forming carbonic acid, which helps regulate the pH and create a suitable environment for cell growth.
Photosynthesis Studies: Photosynthesis is a fundamental process in biology, and CO₂ is a critical component in this process. Plant researchers often study the effect of varying CO₂ concentrations on photosynthesis rates and plant growth in controlled environments or growth chambers.
Respiration Studies: CO₂ is a byproduct of cellular respiration in both plants and animals. Researchers study respiration rates and metabolic processes by measuring the production and exchange of CO₂ during various physiological conditions.
Carbon Dioxide as a Signaling Molecule: In recent years, scientists have discovered that CO₂ can act as a signaling molecule in various organisms, influencing physiological responses and behaviors. Understanding these signaling pathways is an exciting area of research in biology.
Ecological Studies: Carbon dioxide is a key player in the global carbon cycle and climate change. Biology PhD students interested in ecology may study the impact of CO₂ emissions on ecosystems, carbon sequestration in plants, and the responses of different species to changing CO₂ levels.
Aquatic Biology: CO₂ concentration affects the pH of aquatic environments, influencing the survival and behavior of aquatic organisms. Researchers may investigate the effects of elevated CO₂ levels on marine life, particularly in the context of ocean acidification.
Plant Physiology: CO₂ is a crucial factor in determining plant growth and development. Researchers may study how varying CO₂ concentrations impact plant physiology, leaf stomatal regulation, and water-use efficiency.
Immunology and Inflammation: CO₂ has been shown to influence immune responses and inflammation in the body. Researchers may investigate its role in modulating the immune system and its potential therapeutic applications.
Anesthesia and Resuscitation: In medical research, CO₂ is used as an agent to induce controlled anesthesia in animal models. Additionally, it is involved in respiratory physiology studies, particularly related to resuscitation and pulmonary function.
Microbiology and Microbial Ecology: CO₂ is a vital carbon source for many microorganisms. Microbiology researchers may study CO₂ fixation pathways in bacteria and archaea, as well as its role in shaping microbial community structures.
These are just a few examples of how carbon dioxide plays a crucial role in various biological research areas. As a biology PhD student, you may encounter CO₂ in diverse contexts and contribute to the understanding of its significance in living systems.
Thanks for info!
ReplyDeleteThanks a lot ....
ReplyDeleteThank you very much
ReplyDeleteWhy is the structure of carbon dioxide different from the other oxides of group 4A
ReplyDelete"None of the other elements in Group 4 form double bonds with oxygen, so their oxides adopt completely different structures. When carbon forms bonds with oxygen, it promotes one of its 2s electrons into the empty 2p level. This produces 4 unpaired electrons." - https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_14%3A_The_Carbon_Family/1Group_14%3A_General_Chemistry/Oxides_of_Group_4_Elements
Deletehi
ReplyDeletepg auto slot spin เปิดให้บริการ เกมออนไลน์ PG SLOT ที่นักการเล่นเกมสามารถเลือกใช้บริการ PG SLOT สมัคร ฝาก เบิกเงิน เครดิต ผ่านระบบอัตโนมัติเล่นเกมซึ่งนักเสี่ยงดวงทุกคน
ReplyDeleteHi nice reading yyour blog
ReplyDeleteGreatt blog
ReplyDelete