Answer: Hydrogen only forms one covalent bond in order to satisfy its 1s valence shell requirement.
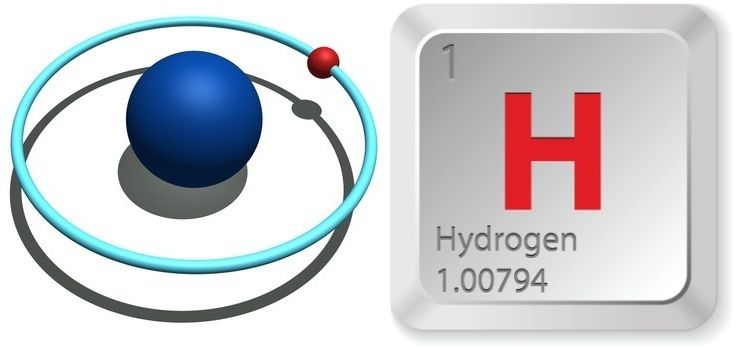 |
Representations of Hydrogen, the first element on the periodic table. Source
|
Hydrogen is the first and smallest element on the periodic table. Hydrogen has
three different isotopes. Click on the link if you want to learn more about isotopes or about hydrogen's isotopes. Two of those isotopes are stable elements while another is radioactive. The one electron which hydrogen possesses is (when in the ground state) located in the 1s sub shell. The 1s shell only contains one orbital and therefore can only hold 2 electrons. Logically we can conclude then that Hydrogen only needs two electrons to fill its outer valence shell. The only other element which this applies to is to is Helium.
 |
| Helium also only needs two electrons to fill its outer valence shell. It already has two electrons in a electrically neutral Helium atom and therefore never forms bonds with any other elements. You can read more about this in our other blog post here. Source |
Covalent bonds involve the sharing of different electrons. Each bond allows for two electrons to be shared. Since this is all hydrogen needs, it can only form one bond. This makes it very useful to cap of different lose ends in molecules. Hydrogen's electronegativity is also relatively average (2.2/4) for nonmetals and metals, which means that it normally forms both
polar and nonpolar covalent bonds. Hydrogen is in its diatomic form (H2) a component of our atmosphere and one of the lightest elements of all time. It has many times been suggested as a
lifting gas, but these efforts have usually failed due to its reactivity with other elements. Since hydrogen is such a small molecule, its electrons easily become exited and it emits high-frequency lightwaves (as shown below).
 |
| Hydrogen in its plasma state. Source |


Thanks for taking the time to explain. I have seen so many students who do not understand hydrogen bonding concepts at once and I think by reading your blog students do not need the second time to understand this concept because you have explained very well.
ReplyDeleteThank you for sharing this post, this helped me understanding the hydrogen bonds which I found one of the difficult topic in science.
ReplyDelete