Xenon tetrafluoride is in fact one of the first molecules ever discovered that involved a reaction with a noble gas. As we've discussed before noble gases very rarely form bonds with other molecules. This makes this molecule quite unique and derives its properties from the large amount of shielding of the valence electrons on the xenon atom. In fact the reaction is exothermic and spontaneous, driven by fluorine's large electronegativity (3.98). Due to the pull the molecule forms a crystal lattice solid structure that doesn't even have a liquid form. It "sublimes" at a temperature of 117°C, meaning that it moves directly from the solid phase to a gas.
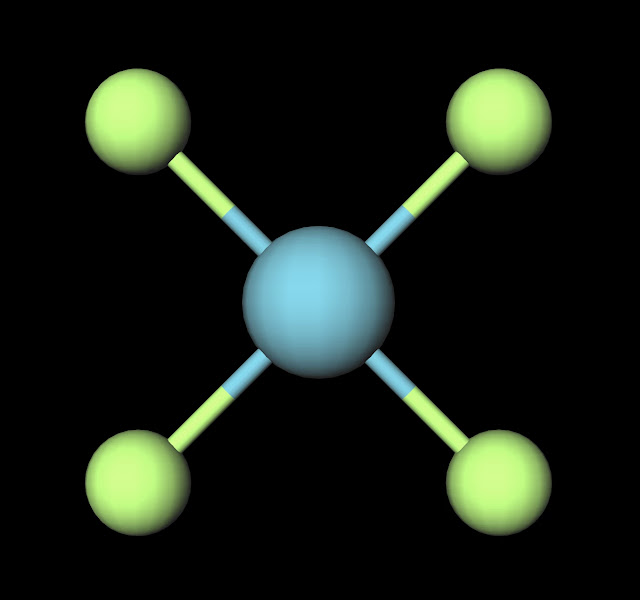 |
| XeF4 Ball and Stick Model. Created with MolView. |
There are few uses of XeF4 but most are related to the presence of four fluorine atoms and their properties as we've discussed with other similar molecules such as NF3 and SF6. These molecules have also been noted typically as severe greenhouse gases that threaten the ozone layer. XeF4 is not measured in this regard because of its higher melting point. One example use is the reaction of XeF4 with silicon products in order to detect metal impurities. In these reactions the only solid products left are the metal impurities which is why this application works. Furthermore, XeF4 reacts with water to form elemental xenon, oxygen, hydrogen fluoride and even xenon trioxide.
No comments:
Post a Comment