In a similar manner as SF4 the more electronegative chlorine atoms (3.16) remove the effect of the lone pair on the dipole moment. However since chlorine is not as electronegative as fluorine this effect is not as extreme. Therefore phosphorus trichloride has a melting point of -94°C and a boiling point of 76°C. This means that the compound is a liquid at standard temperature and pressure. It is likewise a toxic liquid as it is volatile and will quickly react with water or water vapor. Extreme caution is recommended when handling.
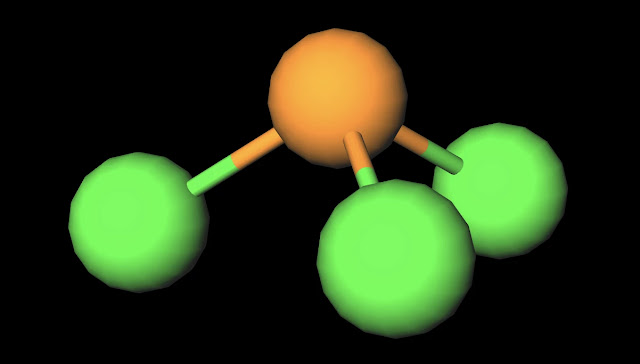 |
| PCl3 Ball and Stick Diagram. Created with MolView. |
Phosphorus trichloride is an important precursor molecule for many other organophosphorus compounds that serve as herbicides or insecticides in addition to many other specific applications. It can also be utilized in chemical reactions in order to add just chlorine to different structures such as alcohols. The molecule can act as both an acid and a base, producing many different strong and weak acids such as hydrochloric acid. At the same time, the lone pair electron permits PCl3 to act as a base and serve as a proton-acceptor.
No comments:
Post a Comment