Although the species has zero dipole moment, the presence of an incomplete octet on this species as with other similar molecules such as BF3 and BH3 leads to an inherent instability within the different parts of the compound since Boron lacks a complete octet. This tension is exacerbated by the presence of the incredibly electronegative chlorines (3.16). This causes there to be an unequal distribution of charge within the molecule between the center and the outer edges. As a result BCl3 has a melting point of -107˚C and boiling point of 13˚C. This means that the compound is a gas at standard temperature and pressure. Due to this unequal distribution of general charge the compound is also soluble within slightly polar compounds such as ethanol of compounds with similar distribution of charge dispersals such as CCl4.
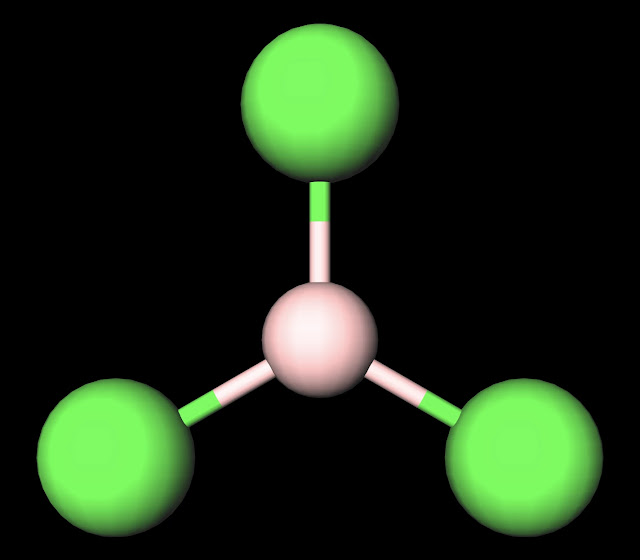 |
| BCl3 Ball and Stick Diagram. Created with MolView. |
Many of Boron trichloride's main uses stem from its properties as a strong acid due to the incomplete octet on the central Boron atom. Since it is quite reactive as an acid, it is often utilized to refine a wide variety of metals including Aluminum, Copper and Zinc. In many of these reactions Boron is the true agent and therefore BCl3 is really a source of Boron. The gas is also reactive enough to serve as a component of certain rocket fuels. Furthermore, it is utilized in organic reactions as a reagent in the synthesis of certain organic compounds. However it is noted that the compound should be handled with care because BCl3 will react with water and certain other alcohols to form hydrogen chloride.
No comments:
Post a Comment