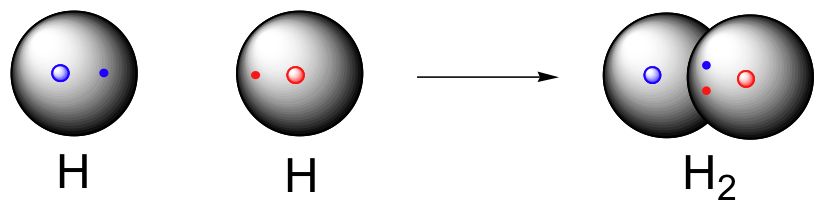
 |
| Representation of the covalent bond joining two hydrogen atoms in the H2 bond. Source |
As we mentioned before, hydrogen can only form one covalent bond because its 1s electron orbital holds a maximum of two electrons. Hydrogen can form either a polar covalent bond or a nonpolar covalent bond (want to know the difference? Click here). H2 (shown above) is an example of a nonpolar covalent bond (because there is no electronegativity difference between the two molecules). Water (shown below) contains polar covalent bonds because the oxygen has a greater electronegativity than the hydrogen atoms.
| Water is a good example of hydrogen bonded in a polar covalent molecule. Source |
Why can I not call this "hydrogen" bonding?
Hydrogen bonding refers to a phenomena which occurs when an H is bonded to either nitrogen, oxygen or fluorine. Water (shown above) is a molecule which can exhibit hydrogen bonding. Due to the large electronegativity difference, strong dipoles are created on either end of the molecule (as shown below). These can then attract one another and create substances with higher boiling points.
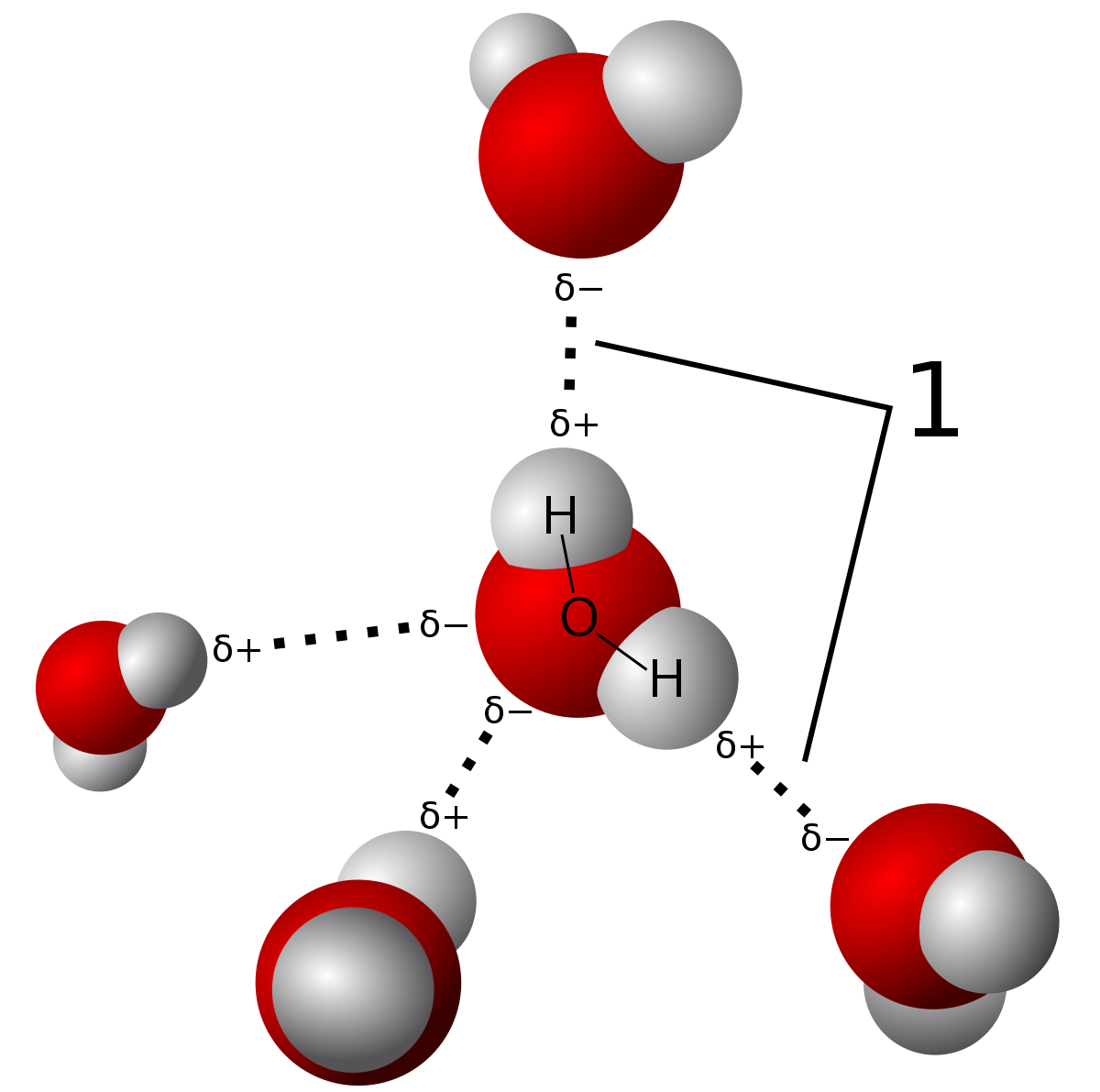 |
| Hydrogen bonding between water molecules. Source |
Therefore, hydrogen atoms are joined to themselves or other elements with covalent bonds.
546466+5
ReplyDeleteHow does the h2 bond exist when the electrons are of same nature
ReplyDeletewell define article thanks
ReplyDelete